|
An improved understanding of the
structure and physiology of peripheral nerves has
led to great advances in the assessment and
management of peripheral nerve injuries over the
past few decades. This knowledge has been
accumulated over the past 170 years, starting with
Schwann's descriptions of the cells named after him
in 1839 up to the sophisticated ultrastructural
studies of recent years.
Peripheral nerves are unique
structures that travel over long distances from the
spine to the skin, muscles, and viscera. Because of
the elongated course of these structures, they are
more susceptible to trauma in many areas along their
courses. Nerves anatomically and physiologically
have evolved to minimize disruption of their
function (i.e. to conduct an impulse to or from the
neuron in the spinal cord or nerve root ganglion).
 Gross Anatomy:
Gross Anatomy:
The cranial nerves and spinal nerves
leave the central nervous system in pairs at
specific levels of the nervous system, usually in
relation to specific anatomic bony structures. The
cranial nerves traverse bony foramina in the base of
the skull before emerging peripherally. The spinal
nerves go through intervertebral foramina. The
nerves within the dura are termed nerve roots and
vary in structure somewhat from the more peripheral
nerve. The spinal roots are divided into an anterior
motor root and a dorsal sensory root. These coalesce
near the point where the root exits through the
dura. The roots differ from the more peripheral
portions of nerves in that they are not invested
with the large amount of connective tissue that is
present distally.
After leaving the dura mater, the
spinal roots in the cervical and lumbosacral regions
join together into plexuses, which rearrange the
course of many of the nerve fibers into identifiable
peripheral nerves. These nerves then follow
well-known anatomic pathways into the extremities.
The cranial and thoracic nerves generally do not
involve themselves in plexus formation and can be
traced from the skull or the spine to their
destinations.
The roots, plexuses, and peripheral
nerves branch at various levels, sending fibers to
specific muscles along their course and receiving
sensory fibers from sensory endings in the skin,
muscle, and viscera. These branches generally follow
a fairly consistent pattern on joining the nerve
trunk, but this can be variable. This pattern of
branching has been helpful to clinicians assessing
nerve function following injury and is one of the
anatomic bases for electromyographic evaluation of
nerve injuries.
The long course of the peripheral
nerves makes them susceptible to damage from
movements of the limbs. Areas of greater
susceptibility exist in most peripheral nerves, and
these areas of entrapment are well known clinically.
In the upper extremity, the median nerve is
entrapped as it traverses the wrist underneath the
transverse carpal ligament. Less known but equally
damaging is compression of the nerve at the ligament
of Struthers at the distal extent of the humerus.
The anterior interosseus branch may be caught in the
pronator teres or in the fascia of the flexor
muscles in the forearm. The ulnar nerve may be
entrapped at the cubital tunnel or in the groove in
the elbow, where it is also susceptible to trauma.
Another area of entrapment is found at the wrist in
Guyon's canal. The radial nerve is most susceptible
to injury in the spiral groove of the humerus, where
it is in close apposition to the bone. It also may
be bound down as it makes a sharply angled dive to
become the posterior interosseus nerve just below
the elbow. In the lower extremity, the peroneal
nerve lies very close to the head of the fibula in a
superficial position, allowing it to be traumatized
quite easily. It also is bound with fibrous tissue
to some extent at this point. The nerve also is
bound at the ankle. This is probably of little
clinical importance, however. The posterior tibial
nerve enters the arch of the foot through the tarsal
canal, made up of ligaments of the arch and
underlying bone, and is subject to trauma in this
region. The sciatic nerve can be fixed in the
sciatic notch, especially with marked flexion of the
hips when squatting (hunkering). The sciatic nerve
also pierces the piriformis muscle in a significant
number of persons and may be compressed at that
point. The femoral nerve is most susceptible as it
enters the femoral triangle in the groin area.
 Anatomy of the Nerve Trunk
Anatomy of the Nerve Trunk
Nerve
trunks are made up of axons, Schwann cells, fibrous
tissue, and vascular components. The ratio of neural
tissue to supportive tissue is variable. Generally,
connective tissue predominates, more so in areas
where the nerve is in apposition to bone or joints,
in areas of potential entrapment, or where the
extremities are most movable.
The axons and their associated
Schwann cells are coalesced into fascicles within
the connective tissue matrix. The fascicles may be
numerous or sparse in a nerve and are arranged
variably from one area of the nerve to the next. In
addition. the pattern of fascicular arrangement
varies from nerve to nerve and also between
individuals. Nerve fibers may change from one
fascicle to another throughout the length of the
nerve trunk.
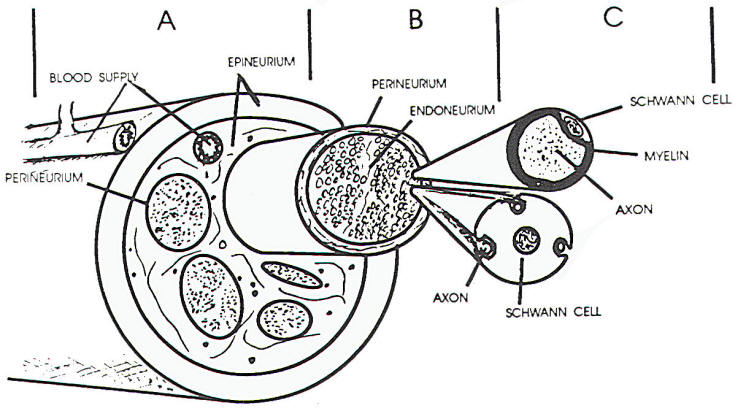
The connective tissue matrix in which
the fascicles lie has been divided into an
epineurium and perineurium, within the fascicles,
connective tissue is less obvious and is termed the
endoneurium. The epineurium is a loosely organized
sheath of connective tissue surrounding the nerve
that also separates the fascicles within the nerve
itself interfascicular epineurium) (Figure 1 A, B,
C). The collagen associated with this connective
tissue is generally arranged longitudinally, though
the interfascicular epineurium may have some
collagen fibers that traverse the nerve, This tissue
provides protection, tensile strength. and supports
the blood supply to the nerve. The outer portion of
the sheath is relatively dense compared to the more
inner regions, allowing for greater structural
support (this is most useful in suturing cut
nerves). The major blood vessels supplying the nerve
lie in the epineurium.
The perineurium is a thin but dense
layer of connective tissue arranged circularly about
the nerve fiber fascicles. The cells lie in layers
bounded by basal lamina on each side. Cells within
the same layer have tight junctions between them and
connections between various layers of cells are
observed. The perineurium extends to the nerve
endings. In the nerve root, the pia-arachnoid
invests the fascicles. In this region, it is
analogous to the perineurium. The tight junctions
and layered structure of the perineurium serve, in
part, as a blood-nerve barrier, resisting the
penetration of substances through the perineurium.
The endoneurium consists of
fibroblasts with processes that disseminate through
the fascicles between nerve fibers and Schwann
cells. The collagen fibers observed in the
endoneurium tend to be longitudinal and often are
closely apposed to the Schwann cells. This close
relationship of endoneurium and Schwann cells helps
form the tube through which regenerating nerve may
pass following nerve injury.
These connective tissue structures
serve to support and protect the underlying nerve
tissue. They provide resistance to stretching, have
some elastic properties, provide protection from
penetration, and help dissipate compressive forces
on the nerve, A nerve may, therefore, be stretched
without impairment of axon integrity. Tolerance to
stretching may vary, in part due to nerves tested,
relationship to points of entrapment, and the
condition of nerves studied. Generally, the nerves
may be stretched up to about 25% to 30% before the
axon is damaged.
 Anatomy of the Nerve Fibers
Anatomy of the Nerve Fibers
The nerve fibers (axons) are
contained in the fascicles. surrounded by the
endoneurium and processes of the Schwann cells.
Nerve fiber diameters vary from 20
µm down to under
1.5 µm. Fiber
diameter diminishes as the nerve proceeds distally
and also is variable from point to point along its
course. The larger fibers are myelinated, whereas
the smallest fibers are embedded in the Schwann cell
walls (Figure 1 C). When viewed longitudinally,
myelinated fibers have indentations in the myelin
(nodes of Ranvier), which are the borders between
adjacent Schwann cells. The axon is exposed in this
area for a very short distance, but the exposed area
is most critical for propagation of a nerve impulse.
Schwann cell nuclei and cell bodies cover the myelin
and. in turn, are covered by endoneurium. The axon
is narrowed at the nodes and occasionally at other
areas, such as under Schwann cell nuclei or other
intracellular material within the Schwann cell.
Unmyelinated fibers do not show the nodal pattern
and are invested by Schwann cell processes. One
Schwann cell may incorporate one or more small nerve
fibers within its endoneurial tube.
|
 |
|
Fig-1 |
Axons may branch along the course of
the nerve, usually distally. This allows one neuron
to innervate widely separated regions. Axon
reflexes, such as the triple-flare response, may be
explained by such branching, as might referred pain,
though there is also evidence that referred pain may
be a more central phenomenon. Nerve fibers lie very
loosely within the fascicles. This allows some
movement within the fascicle but also allows the
nerve trunk to be moved or stretched without
stretching the axons significantly. The connective
tissue structures also tend to be lax, allowing much
of the same protection against stretch injury.
 Blood Supply of Nerves
Blood Supply of Nerves
The blood supply of a nerve trunk.
consists of a network of longitudinally oriented
arteries within the epineurium and over the nerve
sheath. These arteries periodically receive branches
from arteries in the surrounding tissues. forming an
arborization similar to that observed in the
mesentery of the bowel. If one of these nutrient
arteries is damaged, as happens in surgical
mobilization of the nerve, there is still an
adequate blood supply in the nerve through these
longitudinal anastomoses. Mobilization of a nerve up
to 11 cm has not shown significant impairment of
circulation.
Some interconnections between the
longitudinal arteries then branch to deeper
structures, pierce the perineurium in an oblique
manner, and enter the endoneurial space. The
capillaries in the endoneurium have tight junctions
and form the blood-nerve barrier similar to the type
of barrier seen within the brain. This bloodnerve
barrier is of importance in some of the metabolic
neuropathies, and the breakdown of this barrier in
nerve injuries may be of some importance during
repair. Although the basic metabolic support of an
axon comes from the cell body, there is considerable
evidence that the endoneurial blood supply is very
important to maintain axonal function. In clinical
situations where the blood supply to a nerve has
been restricted, symptoms have occurred.
 The Schwann Cell
The Schwann Cell
The Schwann cells have an intimate
relationship with the axons. They probably have a
trophic effect on the axons, help nourish the axon,
and help form the "tube" through which the axon
travels. The origin of these cells is disputed, but
most feel that they migrate from the neural crests
along with the axons. The Schwann cells are the
source of the myelin in peripheral nerves, analogous
with the oligodendroglial cells of the central
nervous system. Myelinated axons are invested in
myelin by a spiraling of a Schwann cell process
about them. Nonmyelinated fibers lie embedded within
a Schwann cell. Often such a cell may be surrounding
several such axons (Figure 1 C). With axonal death,
myelin is destroyed, but the Schwann cells survive
and frequently increase in numbers. If the axon
regenerates, the Schwann cell reinvests the axon,
and forms myelin if needed.
 Physiology
Physiology
Transmission of a nerve action
potential is dependent on the integrity of the
axonal membrane, Damage to this membrane will
interfere with normal neural function. In the steady
state, this membrane has a transmembrane electrical
potential of about -70 to -90 mV with the inside of
the axon being negative.
The reason for this potential
difference lies in both the structure of the
membrane and the distribution of the solutes in the
intracellular and extracellular spaces. The cell
membrane is composed of a double layer of
phospholipids with protein molecules scattered over
the surface but also forming transmembrane channels
for ions to cross the membrane. The membrane acts as
a semipermeable membrane that allows some molecules
to cross it while restricting others. Nerve membrane
is quite permeable to K+ ions, Cl-
ions, and less so to Na+ and other larger
ions. Intracellular K+ concentration is
markedly higher than that found outside the cell. If
the K+ were free to diffuse across the
membrane, there would be an efflux of the ion. The
high extracellular Na+ would tend to try
to get into the cell, where Na+ is low.
The membrane is less permeable to this ion, so less
of a flow is present. The negative potential resists
these flows and maintains the stability of the
membrane. Other ions also participate in various
gradients across the membrane and add their
electrotonic forces to the equation, producing the
final resting membrane potential. The transmembrane
potential of K+ is very close to the
actual resting membrane potential. In addition, an
energy-dependent Na+-K+ "pump"
moves Na+ ions out of the cell and K+
into the cell, maintaining the relative
concentrations within the cell. When a chemical or
electrical stimulus is applied to this system, a
series of events occurs that terminates in the
generation of a nerve action potential. Such a
stimulus needs to reverse (or depolarize) the
negative polarization of the membrane in order to
develop the action potential. When a critical level
of depolarization is reached, there is a sudden
reversal of polarity of the membrane to about +30 -
+40 mV and an action potential is formed. Each time
that threshold is exceeded, the same amplitude of
reversal occurs (the "all or none response").
Associated with this event is a sudden, brief change
in membrane permeability of Na+ that
flows into the cell. About 1 millisecond later, a
similar but longer-duration change occurs in the K+
permeability, which acts to end the action potential
and repolarizes the membrane. During these brief
periods of increased permeability, very few Na+
ions actually enter the cell, but the Na+
- K+ pump will work to remove those few
ions from the internal milieu.
When
the action potential is generated, a current flows
into the active areas of the membrane of the axon
from the extracellular space. This flow then goes
down the axon and exits the axon across the normal
surrounding areas of the membrane into the
extracellular space, completing the circuit. If the
electrical changes in these normal regions exceed
the threshold levels, then a new action potential is
generated and the action potential is propagated
down the axons by way of these local circuits. In
unmyelinated fibers this process is relatively slow;
however, the addition of myelin speeds up this
process considerably. With the insulation provided
by the myelin sheath not allowing the exit of
electrical current except where it is absent (nodes
of Ranvier), the flow of electrical current leaves
the axon at some distance from the action potential
(one to three nodes away). A new action potential is
thus generated much farther down the nerve, allowing
it to propagate down the nerve at a much faster rate
(saltatory conduction). The longer the internode
distance, the more rapidly the axon will conduct the
action potential.
It should be noted that the
metabolism in an axon is greater in the nodal
regions. Mitochondria are grouped in these regions,
providing for the energy needed to sustain the Na+-K+
pump. The propagation of an action potential
requires no energy, but maintenance of the resting
membrane potential does.
Axon metabolism, in part, depends on
substances produced in the cell body, which are
conveyed distally by axoplasmic flow. Both a slow
and a fast transport system occur down the axons,
and, in addition, there seems to be a flow in the
opposite direction. There probably are some Schwann
cells and endoneurium contributions to axonal
metabolism. Certainly, oxygen and carbon dioxide
gaseous exchange occurs in the nodal areas, as
vascular occlusion of the vasa nervorum will cause
malfunction of the axon.
 Clinical Electrodiagnosis
Clinical Electrodiagnosis
Electrodiagnostic tests are an
extension of the bedside examination of the
peripheral nervous system. They add objective data
about the function of the peripheral nerve and
should provide accurate localizing information if a
nerve is damaged. These tests are useful when minor
changes are unable to be identified clinically or
when the functions tested are in locations that are
difficult to examine clinically. They shed light on
pathophysiologic mechanisms that otherwise would be
difficult to delineate at the bedside (e.g.
differentiating neuropraxia from a more severe
injury to the axon, or delineating sensory nerve
root involvement from a plexus injury).
Clinical electrophysiologists have to
be well versed in neuroanatomy, topographic anatomy,
and nerve physiology to make meaningful assessments
of nerve function. The procedures require discrete
placements of the recording electrodes, needles, and
stimulating probes to be accurate. Inaccurate
placement of either the stimulating or recording
electrodes greatly diminishes the value of the
studies. In addition, knowledge of the disease
processes affecting peripheral nerves is of great
importance to the examiner in order for him or her
to interpret the test findings in the proper context
of the nerve dysfunction. Clinical
electrophysiologic testing of the peripheral nervous
system can be divided into two broad categories: (1)
nerve conduction studies with their related studies,
somatosensory evoked responses, and long latency
reflexes (H-reflex, F wave); and (2)
electromyography (EMG).
 Nerve Conduction Studies
Nerve Conduction Studies
The function of the peripheral nerve
is to transmit an electrical impulse from one point
to another. The electrical stimulus normally comes
from the nerve cell body or from receptor
structures. In nerve conduction studies, however,
the nerve is stimulated by an external electrical
source. When the nerve is near the surface of the
body, skin electrodes may deliver the shock. Deeper
nerves require needle electrodes. With nerves
exposed at surgery, stimulating electrodes may be
applied directly to the nerves. Stimulation is made
with supermaximal shocks to make sure that all nerve
fibers are stimulated and that a maximal response is
obtained. Less than maximal stimulation may give
spurious results.
Recording electrodes may also be
surface, needle, or directly applied types. They may
be placed over muscle to record the evoked muscle
action potential, or they may be applied directly
over a nerve to record a nerve action potential. In
sensory nerves, the potential is purely a sensory
nerve action potential (SNAP), but over a nerve
trunk, elements of both motor and sensory nerve
action potentials are present (mixed nerve action
potential). Conduction velocities measure the
fastest conducting fibers of the nerve.
Motor nerve conduction studies are
done by stimulating the nerve at two or more points
along the course of the nerve and measuring the
evoked motor responses from an appropriate muscle.
If the nerve length can be measured between the
stimulus sites, conduction velocities can be
calculated, various segments along the nerve may be
tested, allowing for greater precision in
identifying an area of dysfunction. Motor nerve
conduction velocities vary from nerve to nerve but
generally are comparable from side to side:
therefore, it is most helpful to have information
from the "normal" nerve on the opposite side to
compare with the target nerve being evaluated. Exact
normal velocities expressed in meters per second
vary somewhat from lab to lab but generally are
similar.
Sensory nerve conduction studies may
be performed in two ways. A stimulus may be applied
distally to a pure sensory nerve and recorded
proximally (orthodromic) or to a nerve trunk and
recorded distally off of the pure sensory branch
(antidromic). Both methods achieve comparable
results, though antidromic stimulation may elicit
motor responses that may obscure the smaller sensory
response. Like motor conduction studies, comparison
with the other side is often helpful.
Conduction velocities are only part
of the information that can be obtained from the
test. The amplitude of the response, whether motor
or sensory, is a reflection of the numbers of axons
that are conducting an impulse. Lowamplitude
responses suggest problems with or loss of axons
between the nerve cell body and the site of
recording. The presence of normal sensory nerve
action potentials in the presence of severe sensory
loss points to a lesion proximal to the dorsal root
ganglion, suggesting an avulsion of a nerve root.
Somatosensory evoked potentials
(SEPs) are most helpful in evaluating the proximal
segments of a peripheral nerve that normally are
inaccessible to conventional nerve conduction
studies. A stimulus is usually applied to a nerve
peripherally, and recordings of potentials are made
from proximal nerve sites, areas of entry into the
spinal cord, sites on the spinal cord, and more
proximal areas within the brain. SEPs, therefore,
allow evaluation of the entire sensory system.
Proximal nerve segments, therefore, can be compared
with the more peripheral segments. SEPs should be
performed unilaterally and also simultaneously for
comparison between the two sides.
The H-reflex, first described by
Hoffmann, is the electrical evocation of the spinal
monosynaptic reflex. It therefore allows for the
assessment of both proximal sensory and proximal
motor nerve pathways. It is best elicited from the
calf muscles but also is seen in the flexor carpi
radialis. The stimulus in the leg is applied to the
posterior tibial nerve, allowing evaluation of
conduction in the sciatic nerve and in the S1 root.
In the arm, the median nerve, the lateral cord and
upper trunk of the brachial plexus, along with the
C6 and C7 root, may be assessed with the H-reflex.
F waves measure the motor conductions
along the proximal portions of the nerve. The
stimulus impulse travels toward the cord in the
motor axon (antidromic). Upon reaching the motor
neuron in the anterior horn, it reverses itself and
goes peripherally along the same axon to the muscle
(orthodromic). Unlike the H-reflex, which can be
elicited only in a few nerves, the F wave response
may be obtained from any accessible motor nerve.
Nerve conduction studies may be
affected by numerous factors. Nerve conduction
velocities are faster in larger nerves and those
nerves that are myelinated. They tend to be faster
in the proximal segments than distally. Higher
temperatures may increase conduction velocities.
This, in part, may account for the above
observation, Conversely, cool temperatures slow
conductions, giving the impression that nerve
conduction velocities are slower in wintertime when
the extremities tend to be colder. Constant
temperature conditions in the examining room
minimize these effects. Age affects conduction
velocities, with infant velocities being low and
speeding up to adult levels at about 3 years of age.
Ischemia within a limb also may slow conduction.
The greatest slowing in conduction
velocities occurs with demyelinization or
compression of the nerve, or both. Neuropraxia and
nerve lacerations abolish nerve conduction across
the lesion; however, after a neuropraxic lesion, the
distal segment remains excitable and conduction
remains normal. After a transection, the distal
nerve may remain excitable for 4-7 days after the
injury and then stop functioning. Reports of nerve
conduction studies should include (1) distal latency
(the time required to elicit a response in the
distal most studied segment of a nerve); (2)
amplitude of the elicited response (as noted
previously, this gives some idea of the numbers of
functioning axons within the nerve); (3) conduction
velocities (this is the rate of transmission of an
impulse between two points on a nerve. The segment
being tested should be indicated in the report); and
(4) normal ranges for the lab performing the test
(standard textbooks of electrodiagnosis often
contain tables of normal values for reference where
the norms are not otherwise available).
 Electromyography
Electromyography
EMG tests the electrical activity of
muscles and indirectly the function of both the
upper motor neuron system and the lower motor
neuron. Defects anywhere in this pathway will alter
the EMG findings. The basic unit of muscle activity
is the motor unit. This consists of a variable
number of muscle fibers innervated by one neuron.
When the neuron transmits its impulses, all of its
component muscle fibers are activated and an
electrical potential is generated. This potential
represents the summation of electrical events in the
individual muscle fibers within the motor unit and
can be recorded by an electrode placed nearby.
Needle electrodes are used and multiple locations
must be sampled within each muscle in order to
assess the numbers of motor units in the target
muscle. When a needle is inserted into a normal
muscle, a brief burst of electrical activity occurs
that subsides immediately. This "insertional
activity" may be altered by both denervation and
muscle disease. It may be helpful in differentiating
between them. The muscle should be observed next in
the relaxed state. In normal muscle, no electrical
activity occurs at rest. Denervated muscle will
demonstrate fibrillations and positive sharp waves
as individual muscle fibers become hyperexcitable
and discharge spontaneously. The muscle is examined
next during increasing volitional movement. Motor
unit potentials appear with minimal activity.
As strength increases, new motor
units will be recruited until, ultimately,
individual motor units cannot be identified
(interference pattern). Denervation decreases the
numbers of motor units available for recruitment or,
if complete. will show no motor unit activity. There
also may be changes in the form, amplitude, and
duration of individual motor units as the result of
denervation. Muscle disease also may alter these
parameters of motor unit potentials that are
observed. Reports generated by the EMG should
reflect information from observations in all four of
the preceding areas of assessment.
The EMG requires knowledge of
derivation of nerve fibers going to each muscle.
Nerve fibers in the nerve roots pass through
plexuses and may go to a large number of muscles
through various peripheral nerves. When evaluating
injury to the peripheral nervous system, muscle
should be tested in a logical sequence in order to
determine the location of the lesion. Evaluation of
a nerve root lesion should include EMG of the
paraspinous muscles, as these muscles are innervated
by the posterior ramus of the spinal nerve that
branches at the nerve root.
Following nerve injury, the EMG
changes of denervation will not be present until 2-3
weeks have elapsed. With this in mind, EMG
investigation should not be attempted until 3 weeks
after an injury if one is to obtain full benefit
from the examination. This wait also allows soft
tissue changes to resolve in order to better
appreciate the location of muscles and the nerves to
be tested. EMG should be done with great care in
anticoagulated patients and probably should not be
done in patients with infections in areas through
which the needle electrodes might traverse. No other
contraindications to this procedure exist. |