|
TABLE-2 Relation of Hypertension
to Location |
| |
Hypertension 75 to 50% + |
N0 Hypertension 25 to 50% - |
|
Basal ganglia |
65% |
35% |
|
Subcortical white matter |
45% |
55% |
|
Thalamus |
75% |
25% |
|
Cerebellum |
62% |
38% |
|
Pons |
90% |
10% |
Many
other factors can contribute to bleeding. Damage to the
parenchyma may compromise support to the
vessels:
the significance of this
is not clear, however. Acute increases in blood pressure
and flow may also be important, particularly where autoregulation may be compromised (as in trauma), or where
pressure may be above the limits of autoregulation, as in
toxemia. SICH generally occurs during the morning or early
afternoon when a patient is active. Therefore, it has been
postulated that the trigger for bleeding may be a diurnal
rise or an acute increase in blood pressure from whatever
cause. Last, compromise of hemostatic
mechanisms may playa role, as in delayed traumatic SICH, a
bleeding diathesis, or anticoagulant usage.
It had
been thought that the bleeding event is relatively acute.
However, angiograms have shown bleeding for several hours
and, sometimes, even days from onset. In one systematic
study, it was shown that six of eight patients with serial
CT scans had an increase of the volume of their clot of over
40
percent. In
another series, late deterioration was seen in a small
proportion. It seems that most bleeding takes place within
6 h, and clots larger than 5 cm in diameter are most
likely to expand, which fits with the theory of expansion
due to tearing, which would be more likely to occur in a
larger clot. Many authors believe that this secondary
bleeding is a very important mechanism in clot development. Small satellite
hemorrhages, the marginal
hemorrhages of Stemmer, may be due to similar disruption of
more distant small vessels. The pathologic evidence of such
bleeding is the fibrin-platelet masses found within and at
the margin of the clots. Blood pressure (systemic and
local), size and rigidity of the vessel involved, state of autoregulation, state of the hemostatic system and physical
condition of the surrounding parenchyma probably all play a
role in determining the size of the hematoma. A small number
of patients will develop new clots, usually in a different
location.
The
ultimate clinical manifestations of the clot relate to the
speed and volume of the hemorrhage as well as its location.
The
patterns of spread for each location have been described, as
have the clinical manifestations related to location and
extension. A small hemorrhage may dissect along
tissue planes (e.g., a lobar hemorrhage), splitting the
tissue apart rather than destroying it, with limited
compromise and/or with restitution of function when the
blood is absorbed. A very large hemorrhage may explode
into the brain substance, destroying large amounts of
tissue, raising intracranial pressure to the level of the
blood pressure before the bleeding is tamponaded, and
causing herniation of that part of the brain from its normal
position under the falx, through the tentorial incisura, or
through the foramen magnum, depending on the location of the
bleeding. Even if there is not acute herniation, the brain
is plastic and can further deform or "creep" due to pressure
from the original mass. Local pressure and edema,
disconnection and more distant changes in metabolism and
blood flow, are also important.
Blood
may rupture into a ventricle (especially with caudate,
thalamic, cerebellar and pontine hemorrhages) and even
cause hydrocephalus. On the other hand, the rupture may
actually decompress the clot. Blood may also find its way
into the subarachnoid space causing irritation and
hydrocephalus as well. Distortion of the upper brain stem
may also lead to hydrocephalus.
Death
is due to distortion or compression of the brain stem,
development of secondary brain stem hemorrhages, or direct
extension of the clot into the brain stem. With posterior
fossa hemorrhage, there may be direct compression of the
brain stem. Basal ganglia clots of more than 85 ml or more
than 6 percent of the volume of the brain, or cerebellar
clots more than 3 cm in diameter have a poor prognosis if
left untreated.
If the
patient lives, the clot will eventually be broken down and
reabsorbed. A six-phase process based on evolution of the
clot has been described. It includes invasion by
macrophages, development of surrounding edema, and
development of microvessels at the margin of the clot,
followed by quieting of these processes and development of gliosis (Table-3 ). In the case of a larger clot,
this will take many months. A small number of patients will
develop new clots, usually in a different location.
|
TABLE-3 Evolution of a
Spontaneous Intracerebral Hematoma
|
| |
Stages in Development
and Resolution |
| |
Subacute |
Chronic |
|
Source |
Parameter |
Hyperacute |
Acute |
Early |
Late |
Early |
Late |
Ancient |
|
Kirkpatrick and Hayman
|
Histology |
<6h
Evolution of clot |
7 h-3 days Lysis of clot: entry of
rnacrophages: brain edema |
4-10 days Microvessels at margin |
11days- 6 weeks Resorption of edema |
7 weeks-6 months Processes quiet:
lesion contracts: gliosis develops
|
>6 months Contracted glial scar:
stained by hemosiderin |
|
Chaney et al. |
Histology |
0-24 h |
1-7 days
|
1-2 weeks |
2-4 weeks |
>1 month |
|
Williams ct al. |
Changes in hemoglobin |
|
< I week Intracellular deoxyhemoglobin
|
1-2 weeks Intracellular methemoglobin
|
2-4 weeks
extracellular methemoglobin
|
1-6 months
Intracellular methemoglobin :
hemosiderin |
>6 months Hemosiderin |
|
Models
have been used to study various aspects of SICH. In vivo
models mimic the natural dynamic milieu of human hematomas.
But, animals are expensive, their hemostatic systems may be
very different from those of humans, and their brains are
too small to accept clots large enough to use to evaluate
new surgical devices. However, animal studies have
revealed many details about pathologic and physiologic
changes after SICH. They have demonstrated that blood is
irritating to the parenchyma, causing a progressive
hemorrhagic necrosis with edema at the margin of the clot.
This process is fixed by
6 h. Animal studies have also
demonstrated changes in local and distant blood flow and metabolism. And, in animal studies, early evacuation of
the clot has been shown to improve outcome.
In vitro models
using human blood have been helpful in studying lytic drugs
and aspiration devices, but they lack the dynamic setting
of an animal model.
 Supratentorial
Hematomas
Supratentorial
Hematomas
 Statistics
Statistics
Supratentorial hematomas constitute about 80 percent of
SICHs.
Perhaps half of these hematomas
are related to hypertension. Their highest incidence is in the
fifth and sixth decades of life; males may predominate. Table-4 lists their distribution sites. They may be divided
into
gangliobasal and lobar. Gangliobasal hematomas may occur in
the basal ganglia or thalamus. Those in the basal ganglia
may be internal or deep (two-thirds) or external or
superficial (one-third), depending on their relationship to
the internal capsule. This classification may have
considerable surgical significance. Lobar hematomas tend to
be seen in younger patients. One-third are due to
hypertension. Aneurysms and arteriovenous malformations
(AVMs) are frequent causes, as are tumors and coagulopathies.
There is no obvious etiology at the time of presentation in
almost one-quarter of these hematomas.
|
TABLE-4 Distribution of Hypertensive
Hemorrhages |
|
Site
|
Percentage |
Trends of Variation |
|
Putamen |
35-50 |
+ |
|
Subcortical white matter |
30 |
- |
|
Cerebellum |
16 |
- |
|
Thalamus |
10-15 |
- |
|
Pons |
5-12 |
+ |
 Symptoms and Signs
Symptoms and Signs
Presentation is abrupt or acute with an altered level of
consciousness and progression to death within hours to days
in onethird to one-half of cases (although this is not a
certain figure and reports vary considerably. On the
other hand, there may be only focal signs with preservation
of consciousness with small
hematomas. There are subgroupings
for each of the primary sites and degree of hematoma
extension. An excellent grading scheme
based on level of consciousness has been developed (Table-5 ). Initial symptoms may include headache,
nausea and vomiting. Seizures may be present, especially in
lobar hematomas (50 percent or more) and may occur at onset
or later: they may be an ongoing problem. Clinical manifestations relate to site of origin,
direction and extent of further bleeding, secondary
effects and herniation, as well as to ventricular and
subarachnoid extension with hydrocephalus and meningeal
irritation.
|
TABLE-5 Level of Consciousness |
|
Grade |
Criteria |
| 1 |
Alertness or confusion |
| 2 |
Somnolence |
| 3 |
Stupor |
| 4a |
Semicoma without herniation |
| 4b |
Semicoma
with herniation |
| 5 |
Deep coma |
In
putaminal hemorrhages, motor deficits predominate over
sensory abnormalities. Depending on the extent of the
hemorrhage, other
symptoms may include frontal gaze paresis, homonymous hemianopsia, aphasia if the dominant hemisphere is involved,
and hemineglect if the nondominant hemisphere is involved . Caudate hematomas are less common and tend to be
more benign. They do often extend into the lateral ventricle
and cause hydrocephalus. However, some spread into the
adjacent brain structures, which becomes problematic.
Specific symptoms of thalamic hemorrhages include
hemiparesis, sensory deficits, oculomotor and pupillary
disturbances due to extension into the brain stem or
hydrocephalus, a dysphasia characterized by fluctuation and
paraphasia if the dominant hemisphere is involved. and
neglect if the nondominant hemisphere is involved. Thalamic
pain syndromes and hemisensory strokes may be seen. Specific
syndromes have been described for small hemorrhages. Ventricular extension (including
that from paramedian
and dorsal hematomas, size> 10 cm3) warrants a poorer
prognosis.
Lobar
hematomas commonly result from occult vascular
malformations, microaneurysms (some not related to
hypertension), cerebral amyloid angiopathy or occult
tumors (although many patients also have hypertension. These causes might be anticipated based on previous
hemorrhages, enhancement on CT, oval or round shape
(malformations) or subarachnoid bleeding (amyloid
angiopathy). The clinical picture of lobar hematomas
depends on their location and extent. The location of a
headache may indicate the site. Seizures are
more common and coma is less common than with deep clots.
The clinical pictures of all these lesions depend on site of
origin, direction of spread and size: prognosis is better
than for deep clots. An outcome grading
scheme has been developed (Table-6).
|
TABLE-6 Postoperative Evaluation
of Patients |
|
Grade |
Activities of Daily Living |
| 1 |
Well (full work) |
| 2 |
Minimal disability
(work, self-sufficient) |
| 3 |
Partial disability
(semi-self-sufficient) |
| 4 |
Total disability
(bedridden) |
| 5 |
Vegetative |
| 6 |
Dead |
 Diagnostic Studies
Diagnostic Studies
The general laboratory evaluation indicated for SICH may be
extensive. Besides, routine admission studies, there
should be evaluation of the heart, peripheral vessels
and kidneys. The cause of hypertension might be investigated
in patients with elevated blood pressure, It may be useful to screen for hematologic abnormalities:
infectious processes. and vasculitides.
The
most critical tests for the investigation of SICH are CT
or MRI, both for initial diagnosis and for surgical
planning. The presence of primary intracranial lesion,
including tumor and congenital vascular abnormalities must
be kept in mind.
Because of
the high density of blood, hematomas just a few mm in diameter
can be seen on CT. Indeed, recent studies have shown that
many strokes formerly believed to be due to infarction are really
due to hemorrhages. In addition, details about the hemorrhage,
including exact location, size, associated brain shifts,
ventricular extension and secondary hydrocephalus aid in surgical
planning and may provide the means for improving prognostication
and understanding of the pathophysiology involved. A CT grading scheme has been developed for basal ganglionic
hemorrhage (Table-7). If the medial edge of the hematoma is less than 28
mm from the pineal, the posterior
limb of the internal capsule is involved and the prognosis
is worse. A CT grading scheme has also been developed for
thalamic hemorrhage (Table-8). If the lateral edge
of the hematoma is more than 32 mm from the pineal, the
posterior limb of the internal capsule is involved and the
prognosis is worse. Contrast infusion may provide
additional information about primary lesions and may be
indicated in patients (1) less than 40 years of age. (2) without hypertension.
(3) with neurological impairment
increasing for more than
4h. (4) with history of neoplasm, blood dyscrasia,
vasculitis, or bacterial endocarditis, or (5) with blood in
the subarachnoid space or an atypical location or appearance of
the clot.
| TABLE-7 CT Classification of Basal Ganglionic
Hemorrhage |
| Class |
Type |
Criteria |
| I |
External capsule |
Localized at of internal capsule |
| II |
Capsular (C)* |
Extends to anterior limb of internal capsule |
| IIIa |
Cp without V* |
Extends to posterior limb of internal capsule |
| IIIb |
Cp* with V* |
|
| IVa |
Ca* + p without V |
Extends to anterior and posterior limbs of
internal capsule |
| IVb |
Ca + p with V Th* |
|
| V |
|
Extends to thalamus and subthalamus |
|
* V. massive ventricular
hemorrhage. C: capsule. a: anterior. p:
posterior. Th: thalamus. |
| TABLE-8 CT Classification of Thalamic Hemorrhage |
| Class |
Criteria |
| Ia |
Localized in thalamus without V* |
| Ib |
Localized in thalamus with V |
| IIa |
Extends to internal capsule without V |
| IIb |
Extends to internal capsule with V |
| IIIa |
Extends to hypothalamus or midbrain without V |
| IIIb |
Extends to hypothalamus or midbrain with V |
| * V. massive ventricular hemorrhage |
The change in the CT appearance of the
hematoma has been studied extensively. Within hours, the clot becomes more
dense and a ring of low density develops around it which may
represent edema or fluid squeezed out as it retracts. Initially
hyperdense because or high protein content, acute clots are
better seen with CT than with MRI. With time the clot becomes
isodense with liquefaction and
resorption. Small clots (<2 cm) are absorbed
especially rapidly.
Edema dissipates more slowly than clot reabsorb, but this is
difficult to study in detail because the clot itself becomes
more radiologically isodense. Only a small proportion leave
typical slit-like lesions, and in a number there may be no residual
abnormalities. More work remains to fully understand the time course
of these changes and how they relate to the CT
appearance. The high-field and midfield MRI appearance of
SICH has been studied
extensively. We now know that there are many factors involving clotting and breakdown of the
hematoma as well
as the sequences used that relate to its appearance. Because of the chemical and
physical alterations within and around the clot,
characteristic changes in it, appearance in
different sequences also permit its approximate dating. The
appearance of the hematoma center, hematoma periphery, and
adjacent nearby brain hale been looked at systematically.
Acute clots have magnetic characteristics similar to brain on
T1 and T2 sequences, so gradient echo sequences should be used.
They are better seen after a few days, however MRI changes
reflect lysis of erythrocytes, which occurs from the center
outward, and chemical changes in the hemoglobin molecule (oxyhemoglobin,
0 to 12 h: deoxyhemoglobin. 1 to 7 days methemoglobin. 5 days to months: and hemosiderin, 1
week to years). An area of "ring enhancement" develops around the
margins of the clot, probably related to edema, which is maximal by
4 to 5 day, and whose duration is between 3 and
64 days, and then inflammation occurs between 48 and 84
days. There is eventual resorption of the hematoma (weeks) and
resolution of edema (months). One early schema based
theoretically on changes in hemoglobin uses five time
intervals (acute < I week: early subacute 1 to 2 weeks: late
subacute 2 to 4 weeks: early chronic 1 to 6 months: Late
chronic >6 months).
|
Another schema, derived from an extensive review
of the literature and correlated with histologic changes
uses, five slightly different intervals (hyperacute 1 to 24
h: acute 1 to 7 days: subacute 1 to 2 weeks and 2 to 4 weeks: chronic >
1 month). Table-3 describes the time interval, and MRI appearances. It has
been noted, however, that there may be considerable
variability in the appearance of the clot, particularly
early, because of differences in the many complex processes that
contribute to the rapidly changing appearance (Fig-1). One great
advantage of MRI is that lesions
such as AVMs or tumors are visualized better than on CT,
particularly after enhancement with gadolinium. Hemosiderin
remains in the brain after the blood is absorbed and provides
evidence of prior bleeding. Also, the clots can be visualized
in all planes. |
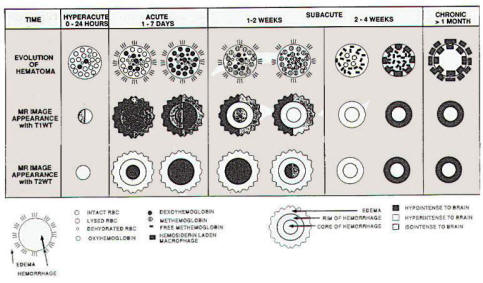 |
|
Fig-1: The MRI appearance
of hematoma on T1 and T2 weighted images.
Note: mixtures indicate that either intensity
has been reported. |
Angiography
may also be indicated if a primary lesion is suspected. It may
be positive in 50 percent of younger patients. Angiography
provides evidence of mass effect and confirms the diagnosis of a
primary lesion such as tumor, aneurysm, or AVM. Because edema
as well as clot can contribute to mass effect, the volume of the
clot may be overestimated by angiography. Conversely, where the
brain is split, the angiographic changes may not fully reflect
the size of the clot.
 Natural
History
Natural
History
Only
one-third of patients present with an abrupt onset. The
remaining patients deteriorate and progression is usually
maximum within hours. Decreased level of consciousness is seen
in 60 percent, with coma in 10 percent. Most who die do
so within a few days .The
patient's subsequent course may be one of deterioration,
improvement, or even improvement with subsequent deterioration.
Comatose patients with large clots can be expected to die.
Overall figures suggest more than 50 percent of hospitalized
victims now survive, which may be attributed both to more
frequent identification of small clots and to improved
treatment. The level of consciousness, the size of the clot,
the presence and degree of shift and evidence of ventricular
rupture are the most important prognostic indicators. Thalamic
clots have the worst prognosis. Unfortunately, older patients
fare worse. Any delay in treatment is harmful. Patients
with marked focal neurological deficits and moderatesized clots
will survive with significant deficits. It is thought that most
survivors are left with deficits, many of which may be
incapacitating. Those with mild deficits and small clots will
recover completely.
 General
Treatment
General
Treatment
For
severely affected patients, comprehensive management in an
intensive care unit (lCU) seems
warranted,
especially to prevent the cardiac and pulmonary
complications that often contribute to death.
Hypertension should be controlled. There is the possibility of
increasing
edema if the blood pressure is too high, and the risk of
compromising cerebral blood flow if the blood pressure is too
low in the face of increased intracranial pressure. The
difficulty in patients with chronic hypertension, however, is
that autoregulation may be altered with regard to the blood
pressure required to sustain flow. It is not yet possible to
individualize the blood pressure required to optimize cerebral
blood flow given generally available technology.
Anticonvulsants, should be used for lobar hemorrhages:
indications for their use in deep clots is not clear.
Corticosteroids are contraindicated since they do not improve
the patient but do cause increased complications.
 Intracranial Pressure Monitoring and Treatment
Intracranial Pressure Monitoring and Treatment
Several studies demonstrated that patients with poor
neurological status had a high ICP. However, some patients
without high ICP did die, presumably from local damage.
Patients with intermediate neurological status did or did not
have increased ICP. Early surgery seemed to help reduce ICP and
improve outcome, but delayed surgery did not. Patients in good
clinical condition had low ICP. ICP monitoring permitted optimal medical
management of the patients, as well, as helped to successfully
guide decision-making regarding whether surgery was
necessary. Ventricular drainage can be beneficial in
treating the hydrocephalus seen in thalamic hemorrhage with
ventricular extension.
 Stereotactic Aspiration with Fibrinolytic and Mechanical
Assistance
Stereotactic Aspiration with Fibrinolytic and Mechanical
Assistance
There are
two purposes for actually removing hematomas: (1) to preserve
life, and (2) maximize recovery of function. Both of these
reasons may be threatened by the mass effect of the clot and
progressive edema and tissue damage. The optimal approach for
removal of an SICH would be a rapid simple method that combines
a high success rate with low risk at minimal cost. One
technique that may prove to
have such characteristics is stereotactic
aspiration.
A number of
features of clots make them suitable for stereotactic
aspiration: (1) they can be easily detected by CT or MRI. (2) they can be localized using stereotactic frames
compatible with CT or MRI. (3) their physical
properties make them susceptible to aspiration with special
devices and this can be
facilitated by instillation of thrombolytic substances, and (4)
their removal may be accomplished without high risk of rebleeding, or under circumstances where bleeding can be
detected (including by intraoperative CT or ultrasonography)
and treated.
Although
the biophysical characteristics of clots and how these change
over time have not been described in detail, early attempts at
aspiration of fresh hematomas were recognized as being
only
partially successful because
of difficulties in removing the more solid components of the
clots. On the other hand, the use of a large (5 mm) cannula for
aspiration with transventricular irrigation of deep clots may at
times be successful. In Japan, aspiration often yielded one-half
to two-thirds of the clot volume. To fully
understand the meaning of this
information would require more knowledge about the inner
diameter of the catheters, the size of the ports, and the amount
of vacuum applied as well as the age of the clot and its
appearance on CT or MRI, and the hematocrit and clotting status
of the patient. Simple aspiration does appear helpful for
medium-sized (22 to
30
mm in diameter) pontine hematomas.
To
facilitate aspiration, a number of devices to physically morcellate hematoma material have
been developed and used in both experimental and clinical
investigations. The first such instrument was a 4mm cannula in which there was an
Archimedes screw. Suction was applied to bring the clot up into
the cannula where it could be broken up by rotating the screw.
The device was used successfully for subtotal removal. It has
been modified by other surgeons and used with some success, but
was never adopted widely. Another device involves high-pressure
fluid irrigation to facilitate
suction-aspiration of
hematomas.
The authors who described the
device suggested restricting its use to hematomas more than 24h
old, due to fears of rebleeding in operations done earlier.
Other sophisticated mechanical approaches, namely breaking down
the clot with ultrasonic aspirators, have been used. With a vacuum of 150
mmHg, it could be aspirated 75 percent of a 4-h old clot in 15 min.
Another
approach has been to try to liquefy clots chemically to make
them more amenable to aspiration. There is a great deal of
activity in the development of thrombolytic drugs. In experimental studies of subarachnoid,
intracerebral and intraventricular injections, urokinase
appeared safe and indeed promoted clot reabsorption. Since
1980.
several Japanese groups have
had extensive experience using urokinase in spontaneous
intracerebral hematomas in humans, including posterior fossa
clots and intraventricular clots. They have indicated that it
can be helpful, although it has been associated with rebleeding
in 4 percent of cases. Tissue plasminogen activator is safe when injected into the
brain of rats and the CSF of rabbits. It also seems to promote
clot absorption
and has been used to dissolve intraventricular clots.
An
endoscope with irrigation, suction, and a laser for hemostasis
was employed in a randomized series of 100 patients and was
thought to be useful in removing subcortical as well as
putaminal and thalamic hematomas. Although in this report the
endoscope was inserted using ultrasonography for guidance, a
similar technique has been reported using stereotactic
positioning of the endoscope.
The
aforementioned information suggests that eventually some form of
stereotactic aspiration will be developed that will provide an
optimum method for evacuating intracerebral hematomas. It is
obvious that more needs to be known about the properties of
clots, particularly regarding their susceptibility to morcellation and lysis at various intervals after formation, and
the coagulation status of the patient. The capabilities of the
various mechanical devices need to be studied in more detail, as
do the fibrinolytic drugs now available and the new and
improved drugs that certainly will be developed in the future.
 Open
Evacuation
Open
Evacuation
Surgery is not indicated in the face of
irreversible neurological damage suggested by great depressed level of consciousness, rapid
clinical deterioration or massive size of hematoma and is
generally not needed in the case of patient, who are alert and
have hematomas less than 2 cm in diameter. Some patients with
clots between 2 and 3 cm may benefit from surgery. Critical
size may also be 85 ml. One suggestion is that surgery
is not needed if the clot occupies <4 percent of the
intracranial space, should be based on the clinical status if 4
to 8 percent, should be done for 8 to 12 percent, and will not
help if > 12 percent. Not all agree on these guidelines, and
there are certainly exceptions: for example, small clots in
critical area can be life threatening and large clots can be surprisingly well tolerated. Some authorities advocate immediate
surgery (<6h) to minimize ongoing bleeding, irritation of the brain,
and edema. Others suggest waiting at least
6 h to minimize the possibility of rebleeding. If patients have not needed surgery
by day 10,
deterioration is infrequent.
Open surgery
has been used for lobar hematomas with considerable success
although hematomas due to amyloid angiopathy are more
problematic. Because of the risk of brain stem
compression, temporal clots as small as 30 ml should be
considered for evacuation. Approaches should be made where
the clot extends toward the surface or through silent areas
of the brain. Surgery for deep clots has been facilitated by the
development of transtemporal and trans-sylvian
approaches.
The general
principles regarding skin, bone, and dura incisions should be followed. Ultrasonography can be used to confirm
localization of the clot. Modern technique includes the
standard use of magnification and good illumination as well as
gentle retraction to minimize difficult-to-control
intraoperative bleeding. Most authors recommend that small
amounts of adherent clot be left undisturbed, although some
suggest that all clot should be removed, which would allow
examination of the entire cavity for evidence of an AVM or
tumor. If there is any question of the etiology of the hematoma,
surgery should include biopsy of the wall of the cavity. An
interesting observation since CT has been available is that,
with early surgery, the mass effect may actually increase after
evacuation.
A number of
developments from the 1970s make only recent series relevant
when trying to understand the potential role of surgery. With
modern neuroimaging, diagnosis is rapid and the anatomy clear.
Medical care in an lCU setting can optimize cardiac and
pulmonary function. ICP monitoring and control (although this
has not been used frequently in SICH patients) is available.
Clinical and CT grading scales as well as outcome scales (Tables-5 to 8) have been developed so that patients can be
compared from series to series. Experimental design uses
randomized clinical trials at best or closely matched controls
in contemporary prospective trials. Even in current reports,
however, information about these factors is not always
available.
Some,
although not all of the literature is encouraging. For
example, on the basis of matched controls (410 surgical
patients vs. 204 medical controls), surgery was helpful in all
patients except those who were alert or only somnolent. Using 165 medical controls for 187
surgical patients, only grade III (i.e., moderately impaired)
patients benefited from surgery. Using internal controls
(N
= 265),others believed that moderate and severe
cases who were operated on did slightly better: they did not
come to a conclusion with regard to thalamic clots (N = 135
). Comparing 44 patients who had surgery for putaminal hemorrhage with 130
who did not, it was decided that
surgery was actually harmful. Kaneko et al., comparing 100
patients with putaminal hemorrhage who had ultra-early
operations with historical controls, believed that surgery was
more beneficial than conservative management or delayed
operations. On the basis of a combined study in Japan using
historical controls. Kanaya et al. believed that surgery for
putaminal hemorrhage associated with stupor, semicoma, and
coma (N = 3216) was definitely helpful. They also recommended
operation for thalamic hemorrhages (N = 639). Juvela et
al. randomized 52 patients to surgery or conservative care. The
only patients who did better with surgery had Glasgow Coma Scale
scores between 7 and 10. However, although several survived, all
were disabled. In a small (N = 21) randomized study of
putaminal hematomas (>3 cm) in hypertensive patients utilizing
transinsular microsurgery, Batjer et al. concluded that
evacuation was not helpful. There were a higher proportion of survivors in those operated on, however, and the early
termination of the study may have truly been premature. Fujitsu
et al. concluded that the most important factor in determining
the need for surgery was the time course in the first 6 h after
bleeding. They believed that surgery was not beneficial for
those with a fulminating course, but it could help those with a
rapidly or slowly progressive course if done before
irreversible damage, and was not needed if the patient was
stable.
Some
authorities suggest delaying surgery 48 to 72 h until the clot
is partially liquefied. The surgery is therefore technically
easier, and the chance of rebleeding is reduced. Also, there
are some patients who either stabilize and then deteriorate, do
not improve, or only improve slowly, and who may benefit from
surgery even up to 4 weeks later to decompress nearby
neurons.
In the largest reported experience, Kanaya and Kuroda continued their
work in the fifth all-Japan study in which 339 institutions
participated. There were 7010 patients with putaminal
hemorrhages studied, of whom 3375 were operated on and 3635
were not. Both aspiration and craniotomy were investigated. Using information from neurological examination, CT grading,
clot volume and deformity of cisterns, it was concluded that
small hematomas did not require surgery, intermediate ones
should be treated with serial aspiration with injection of
urokinase, large ones causing "semicoma" or early herniation
should have open surgery, and terminal patients should be treated
expectantly. They also suggested that thalamic hemorrhages with
hydrocephalus should be treated with ventricular drainage and
possibly open surgery if they extended to the hypothalamus and
midbrain and that lobar hematomas with semicoma benefited from
surgery. More formal and rigorous studies are needed to
define precisely which patients should be managed aggressively
and how to optimize treatment.
 Intraventricular
Hematoma
Intraventricular
Hematoma
Intraventricular hemorrhage may be an isolated (and often benign) problem and may be due to
an AVM of the choroid plexus. However, almost 80 percent of
intraventricular hematomas (IVHs) are related to intracerebral
hematomas, and they are usually caused by hypertension,
aneurysms, AVMs and even pituitary
apoplexy. They are often
accompanied by slightly enlarged ventricles. One-third of SICHs
are accompanied by lVH, and these have a higher mortality rate.
The primary hematoma and disease process are probably of more
significance than the IVH. However, prognosis is also determined
by the extent of the hemorrhage. Headache, vomiting,
confusion, decreased level of consciousness and, in the case of
secondary bleeding, hemiparesis, are common clinical
findings. The clots tend to disappear within 2 weeks. Recent
CT studies suggest that IVH is more frequent than previously
suspected, but it is often not significant clinically. When
clots are symptomatic, intraventricular drainage (possibly
bilateral) may be useful, but the blood often occludes
catheters used for this purpose. Ultimately, a shunt may be
required if permanent hydrocephalus develops. Intraventricular
thrombolytic therapy has been shown experimentally to be
useful and safe and has been used successfully in
humans. Direct
surgery has not proved
useful.
 Infratentorial
Infratentorial
 Cerebellum
Cerebellum
Cerebellar
hematomas constitute about 10 percent of SICH, a proportion
coincident with the volume of brain in which
they occur.
These occur
more commonly in males. The highest frequency is in the sixth
through eighth decades of life. Two-thirds of cerebellar
hematomas are related to hypertension. These usually occur in
the dentate nucleus which is irrigated by the superior
cerebellar artery. There may be a left predominance. A small
number originate in the vermis. Hematomas in the younger age
group may be related to vascular malformations. Anticoagulants
are another common predisposing factor.
Many of
these hematomas are extensive and rupture into the fourth
ventricle and also into the subarachnoid space. Secondary
hydrocephalus may develop in up to 75 percent of patients. Death
occurs in 60 to 80 percent of patients and is due to brain stem
compression and tonsilar herniation.
Symptomatology is related to the rapidity of bleeding and the
size and location of the hematoma as well as to compression of
the brain stem, upward cerebellar herniation and tonsilar
herniation, hematoma rupture into the fourth ventricle, and the
development of hydrocephalus. The onset of symptoms is often
abrupt. but may be subacute with progression over various times,
or subacute with resolution. Symptoms and signs are protean and
include headaches, alterations in level of consciousness,
vomiting with or without nausea, dizziness, eye signs.
including changes in pupils and gaze abnormalities, dysarthria, and motor signs, both cerebellar and
pyramidal. The classic triad of signs includes appendicular
ataxia, ipsilateral gaze palsy, and peripheral facial weakness.
Two out of three of these findings are seen in
75
percent of patients. A
classic three-stage evolution has been described.
The
diagnosis can be difficult if the history is not known and the
patient is stuporous. The differential diagnosis is again
extensive and includes cerebellar infarction, brain stem
hematoma or infarction, bleeding from an aneurysm or a tumor in
the posterior fossa, as well as acute labyrinthitis. Clinical
diagnosis is often difficult. In one series of
33 patients, 13 with
cerebellar hemorrhages or infarctions were diagnosed correctly.
10 were not diagnosed initially, and 10
diagnosed as having cerebellar
strokes actually had other problems.
Diagnosis
can now be readily made using CT, which can also be helpful in
surgical planning. The clot can be well visualized and other
abnormalities including blood in the fourth ventricle, brain
stem distortion, and hydrocephalus can also be seen. If the
patient is not too sick and the test is possible, MRI can
provide evidence of a vascular malformation and previous
bleeding as well as to better define the anatomy. Angiography
can demonstrate mass effect and might be employed if an AVM
or other specified lesion is suspected, particularly in a young
patient without a history of hypertension. (Even if this is
negative, because of the high risk of rebleeding from a vascular
malformation, surgery should also be considered.)
Treatment
includes control of blood pressure and respiratory support as
needed. Surgery involves a posterior fossa craniectomy and
evacuation of the clot. The indications for surgical therapy
are probably better defined in this group of hematoma than in
those in other locations. The key indicator, are based on the
level of consciousness, clinical course, and size
(2 to 3
cm) of
the hematoma, unless the patient is seen after
doing well for a week. All clots 3 cm or larger and those
between 2 and 3 cm (if the patient's level of consciousness is
altered), should be considered for surgery, especially if there
is deterioration, since some patients may decompensate rapidly.
Mortality is 72 percent if the patient is comatose. Most
patients without impaired consciousness will improve
spontaneously. Indeed, CT scans have shown that clots tend to
disappear in 2 to 6
weeks.
In the
past, the use of ventricular drainage by itself was
discouraged for two reasons: (1) it did not address the major
problem, namely brain stem compression, and (2) because of the
risk of upward cerebellar herniation. Indeed, it may delay
definitive treatment and it has been suggested that it
therefore be employed only in conjunction with clot evacuation.
But in cases with clots of borderline size, and possibly in
conjunction with mannitol administration, this may be an
alternate mode of treatment.
Excellent
surgical results with relatively low operative mortality have
been described in patients with only moderately depressed levels
of consciousness. Occasionally, patients with marked
alterations in level of consciousness, particularly if they did
not have too abrupt an onset and if operation was performed
promptly, have improved with surgery. Patients in extremis are
beyond help. Some patients with late deterioration or persistent
deficits may also be helped by evacuation. Stereotactic
aspiration has also been advocated.
Although
the guidelines for surgical treatment of cerebellar hematomas
are probably better defined than for those in other locations,
there may still be questions in those patients who are quite ill
but not in extremis, or who are doing relatively well but are
not improving rapidly.
 Brain
Stem
Brain
Stem
Brain stem
hemorrhages tend to occur predominantly in the pons, although
hemorrhages in the midbrain and medulla have been described. Pontine
hemorrhages constitute about
3 to 13
percent of SICH far out of proportion to the
volume of brain involved. Males and females are equally
affected. The highest frequency is in the fourth and fifth
decades of life. Ninety percent are related to hypertension and
are believed to be due to vascular disease of penetrating
branches of the basilar artery. Those hemorrhages seen in
younger patients without hypertension may be related to cryptic
vascular malformations, which are especially common in the pons
but probably account for less than 10 percent of such
hemorrhages.
Hematomas are present unilaterally in the basis pontis
(at times with progression into the tegmentum) in 22
percent, in the basis bilaterally in 56 percent, and in the
tegmentum in 22
percent, (two-thirds bilaterally). Clots extend upward, even to the
thalamus, but infrequently downward. The fourth ventricle is
usually distorted. There is rupture into the fourth ventricle
in at least 70 percent of cases. Extensive edema is often
present, the cause of which is unknown. Local vascular disease
is common. as is evidence of other cerebrovascular and
cardiovascular disease.
Symptomatology is based on location, size, speed of
development and rupture into the fourth ventricle and
subarachnoid space. as well as hydrocephalus secondary to
ventricular occlusion or compression of the fourth ventricle and
aqueduct. In the large postmortem series, the
onset was abrupt in one-half. In 30 percent, the initial symptom
was severe headache, usually posterior. Symptoms and signs
included alterations in level of consciousness, abnormalities
of respiration, pulse and blood pressure,
hyperthermia, motor
abnormalities that were often bilateral with posturing or
paralysis, cranial nerve abnormalities, including pupillary and
gaze change, with ocular bobbing, vertigo, vomiting, dysarthria,
autonomic dysfunction, and "seizures" believed to arise from the
basis pontis. The classic triad of miosis, hyperthermia. and
bloody CSF was seldom seen. The diagnosis was suspected in
only 25 percent
of the cases. Seventy-five percent of patients died within 24h.
The common
presentation of coma with neurological devastation involves an
extensive differential diagnosis, including massive hemorrhages
in other locations as well as posterior fossa infarcts and
hypertensive encephalopathy. Definitive diagnosis can be
made with CT scanning. The diagnosis may also be made with MRI.
Angiography might be employed if a vascular malformation is
suspected. Mortality is more than 80 percent in 48 h and more in
the first week.
Treatment
depends on the patient's condition. Most patients present with
an acute onset of devastating symptoms and will die. One group
has suggested that ophthalmologic findings as well as the size
and location of the clot may be useful in predicting potential survivors. In patients believed to be treatable, there should
be immediate attention to respiratory support and control of
blood pressure where needed. Ventricular drainage might be used
if hydrocephalus is present, but the very presence of
hydrocephalus may be a marker of a fatal hemorrhage. A major
question concerns the role of direct surgery. On the one hand,
hematomas have been followed by CT and have been seen to resorb,
occasionally with a good result. On the other hand, several
cases, including a few with acute onset, have been thought to
have been successfully operated on either through the fourth
ventricle or subtemporally. CT might suggest the best
route. Biopsy of the wall is thought
to lead to deterioration. Stereotactic aspiration has also
proved helpful. Other series, however, have suggested that
acute surgery does not improve outcome. Patients with
persistent symptoms from unresorbed clots might have direct or
stereotactic aspiration, and patients with recurrent bleeding
due to vascular malformations should be considered for open
surgery, Collaborative studies will probably be needed to define
those patients who are ill enough to require surgery but not
yet beyond hope. Finally,
the usefulness of rehabilitation for many stroke victim appears
well established and must be pursued when the patient is stable.
 Nonhypertensive
Nonhypertensive
 Aneurysm,
AVM
Aneurysm,
AVM
These
anomalies are the second leading cause of SICH and there should
be a high index of suspicion in young patients and those with
superficial hematomas. A history suggesting a
sentinel hemorrhage, or in the case of AVMs. seizures,
headaches, or focal findings may increase the index of
suspicion. It is
thought that of those patients with aneurysms that bleed, 40
percent will have SICH, one-half of these >3 cm in diameter.
Aneurysms bleed into the brain when the aneurysm is typically
imbedded in brain (i.e., internal carotid bifurcation, anterior
cerebral artery, distal anterior cerebral artery),
when it points into the brain
(i.e., posterior cerebral artery), when surrounding structures
are scarred from previous bleeding, or when the local brain is
already damaged. SICH is more common after the first hemorrhage. Aneurysm should be suspected if the clot is
frontal or temporal in location, although even
basal ganglia clots may originate from aneurysms. Aneurysms >5 mm in diameter may be seen when enhanced CT
scans are compared to unenhanced ones, especially with fine cuts
which include the sites of typical aneurysms. Repeat CT may
also be helpful to detect lesions not seen initially because of vasospasm and/or compression which could prevent filling,
Angiography should be utilized aggressively and certainly for
any patient who might be an operative candidate. Repeat
angiography may be necessary if no lesion is initially seen. Early surgery is indicated because of the risk of early
rebleeding, The aneurysm should be clipped during the initial
operation, preferably as the first step using subarachnoid
dissection to initially obtain proximal control. If the
patient is moribund, there may not even be time for an
angiogram, but the CT can provide some information about the
aneurysm. Patient outcomes are worse for aneurysm surgery in
the presence of an intraparenchymal hematoma.
Angiomatous
malformations include AV fistulas, classic AVMs, telangectasias, cavernous angiomas, venous angiomas, and dural AVMs.
These lesions should be considered in younger patients without
hypertension who have hemorrhages that are superficial, lobar, periventricular,
or into the ventricle, clots that have a low
density ring around them, or subarachnoid blood.
Hemorrhage is the presenting symptom in 30 to 55 percent, and 50
to 66 percent of patients with classic AVMs that bleed have SICH.
Ten to twenty percent of AVMs that bleed
have aneurysms, which may or may not be associated with the AVM. Bleeding from an AVM occurs most commonly
from the draining veins or the nidus near the veins, but can arise
from the aneurysm, AVMs with central venous drainage, a periventricular or intraventricular location, and an intranidal
aneurysm may be most likely to bleed.
Enhanced CT will often show the nidus and the draining veins. In
some cases, however, the clot may compress the AVM and prevent
its filling: repeat studies may be required, MRI is more
sensitive for detecting AVMs. Some AVMs and cavernous
angiomas may be occult (i.e., not seen on angiograms)
and studies may
need to be repeated.
Eighty
percent of AVMs can be resected. It is best to delay
surgery to allow neurological deficits to resolve. Also, it is
easier to operate after the brain is less swollen, the AVM is
better seen. and the clot has liquefied. If immediate
surgery is required, a flap should be turned that is large
enough to permit resection of the AVM and clipping of the
feeding vessels. However, if intraoperative bleeding is
difficult to control. it is preferable to only remove the
hematoma and to resect the AVM at a later time, since the early rebleeding rate is thought to be small. The clot should
also be sent for pathologic examination. On the other hand, if
during surgery a cavernous angioma is seen, it should be
resected if this seems easy. The veins of venous angiomas
may drain normal brain, and careful analysis of angiograms is
required to determine if they can be safely resected. Dural AVMs are only rarely the cause of SICH, They can be complex
entities, and their treatment requires special considerations.
 Hematologic Disorders
Hematologic Disorders
Hemostasis is governed by complex interactions among blood vessels,
platelets, and blood coagulation factors. Defects in hemostasis either exacerbate
bleeding from other problems, such as trauma, or they lead to
spontaneous bleeding if severe. Thus, spontaneous bleeding is
most common if platelets are less than 10.000/ml or activity of
a given clotting factor is less than 1 percent of normal.
Abnormalities of the two entities can be classified as follows:
| Platelets
|
|
|
|
| |
Thrombocytopenia |
|
|
| |
|
Peripheral
destruction-immune (i.e., idiopathic thrombocytopenic
purpura) |
|
| |
|
Decreased production (i.e.. marrow injury or
replacement) |
|
| |
Disorder, of
function |
|
|
| |
|
Inherited
|
|
| |
|
Acquired |
(i.e., von
Willebrand's disease) |
| Coagulation
Factors |
|
|
|
| |
Inherited
deficiencies |
Hemophilia |
can be complicated by AIDS, inhibitors |
| |
Acquired
disorders |
involving deficiencies and inhibitors |
|
| |
|
Disseminated intravacular coagulation |
|
| |
|
Liver
disease |
|
It is
important to have a consultation regarding treatment of the
primary disease process, replacement of clotting factors and
platelets, and decisions about surgery based not only on the
acute but also on the ultimate prognosis of the patient. With
the risk of transmitting various diseases, particularly AIDS,
thresholds for prophylactic use of blood products and accepted
replacement levels are
changing.
 Tumors
Tumors
Although intracranial tumors may bleed into a variety of
sites, they most commonly bleed into the
brain, and even more specifically into the tumor. Depending on
the biases of the patient population. tumors may be the
third or fourth most common specific cause of SICH. In one
literature review, tumors caused 4.6 percent of all SICH, and
3.9 percent of patients with tumors had SICH. Metastatic
tumors (especially bronchogenic carcinoma, melanoma, choriocarcinoma or renal tumors) most commonly, but also
gliomas, especially more malignant ones
medulloblastoma and even
benign tumors (meningiomas, pituitary tumor) have been
associated with SICH. Factors leading to bleeding include
hypervascularity, abnormal vessels, invasion of vessels and tumor necrosis as
well as, related disorders of hemostasis. Bleeding
mal occur after needle biopsy, shunting, decompression (even at
a distance), and radiation therapy. The bleeding may be related
to other factors such as anticoagulation or trauma. In many
cases the patient was already known to have a tumor, or there was a prior history of progressive
neurological dysfunction or headache. In one-third of
cases, however, bleeding may have caused the
onset of symptoms, which may have been abrupt or gradual. On
the other hand, many hemorrhages, are small and asymptomatic.
CT abnormalities that may suggest a tumor
include subcortical site,
unusual appearance with abnormally appearing or enhancing tissue
within or adjacent to the clot, and excessive edema or
mass effect adjacent to the clot and extending even across the
midline. Multiple lesions would also be suspicious. An
angiogram may demonstrate abnormal vessels but is usually not
required.
Surgery may be indicated depending on the
clinical significance of the clot
and the nature of the underlying disease. The surgeon should
remove as much of the tumor as possible, not only to treat the
underlying disease but also to minimize the risk of rebleeding.
(If surgery is
carried out for
any clot, any suspicious tissue should he sent for histologic
examination). Patient, often, but not invariably, do badly because
tumor, that bleed are often very
malignant and because the prognosis for a large clot by itself
is often poor.
Pituitary
hemorrhages occasionally develop from a normal gland or
nonadenomatous tumor, but generally arise from adenomas, both active and inactive endocrinologically. Indeed, <
1 to >
12 percent of pituitary adenomas give rise to pituitary
apoplexy. Asymptomatic small hemorrhages are more common.
and even large asymptomatic hemorrhages are seen. The bleeding
may be spontaneous or may be precipitated by trauma,
anticoagulant, estrogen, or bromocreptine usage, or radiotherapy. The etiology may
not be clear. Clinical presentation includes sudden
headache, nausea, stiff neck, decreased vision
and field cuts, and impaired eye movements. CT and particularly
MRI reveal the diagnosis: most hemorrhages extend
above the sella. Steroid replacement and prompt surgery (generally
transsphenoidal) are recommended. If some function is preserved, most deficits;
(except complete loss of vision) improve or
clear even if the patient has been symptomatic for a few days.
 Vasculopathy, Vasculitis
Vasculopathy, Vasculitis
Vasculopathy
includes conditions, that have proliferative changes or
intramural deposits
of
adventitious materials. Vasculitis
includes conditions characterized by inflammation and necrosis
of vessel walls. A variety of classification schemes have been
used. There are a variety of types which may be of
specific or nonspecific etiology which involve the brain only
or are generalized. which may involve vessels of different
sizes, and which have different histologic appearances. They
cause SICH by weakening vessel wall, by occluding vessels
leading to infarction into which bleeding
occurs, or by causing myocardial infarction, cardiac embolism,
and a transforming infarction. The diagnosis is much more
obvious when there is typical systemic involvement. The
diagnosis is made by angiography and biopsy.
Cerebral
amyloid angiopathy (CAA) may be the third leading cause of SICH.
It predominates in the elderly population. Amyloid is deposited
in the media and adventitia of small- and mediumsized
superficial cortical and leptomeningeal arteries that become
brittle and rupture and also lose the hemostatic function of
their endothelia. CAA is expected to be a more common cause
of SICH as our population ages. Seen in 10 percent of those in
their 70s, and in over 60 percent over 90, it leads to recurrent
and multiple superficial hemorrhages from the weakened vessels.
There are familial varieties. It is also associated with a
variety of diseases from Alzheimer's disease to dementia pugilistica, and many patients have hypertension. The diagnosis
must be made by biopsy or postmortem examination. The prognosis
is usually poor, and surgery may be complicated by difficult
hemostasis and rebleeding, although it can be done
successfully.
Fibromuscular dysplasia may lead to aneurysm formation.
Secondary SICH may then occur. Moyamoya
disease is a specific condition or a syndrome resulting from
various diseases causing occlusion of proximal cerebral vessels.
It is characterized by progressive stenosis of the anterior
circle of Willis and compensatory transdural or posterior fossa
anastomoses and collateral channels in the basal ganglia.
Bleeding occurs from microaneurysms in the vessels in the basal
ganglia or secondary proximal internal carotid and posterior
fossa aneurysms. SICH is the most common cause of death.
There are
both multisystem (systemic lupus erythematosus, rheumatoid
arthritis, giant cell arteritis) and isolated (granulomatous
angiitis) vasculitides that can lead to cerebral vascular
weakening and bleeding. The diagnosis can be suspected if the
systemic disease is present: it can be confirmed by a picture of
vascular stenosis and narrowings on angiography.
 Drugs
Drugs
A number of
sympathomimetic street drugs, including amphetamines and
cocaine, as well as over-the-counter drugs, may cause SICH,
generally after chronic abuse. This may be due to hypertension
and/or vasculitis. At times, angiography will demonstrate
vasculitis in the small- and medium-sized arteries. The arteritis will subside with cessation of drug use and the
administration of cyclophosphamide and prednisone. However,
blood pressure elevations per se may also precipitate rupture of
preexisting aneurysms and AVMs. The clots tend to arise in the
subcortical white matter.
Anticoagulants can lead to SICH, especially if the clotting
studies are especially prolonged (i.e., prothrombin time> 1.5
times normal). The hemorrhage may evolve slowly and become very
large. Related to age, hypertension, head injury (even minor),
and infarction, it may be the cause of up to 10 percent of SICHs.
SICH may be seen in up to 2 percent of patients on
anticoagulants. The parenchyma is the second most common site of
intracranial bleeding (after the subdural space) that tends to
occur in the lobar white matter or cerebellum. Treatment
involves normalizing the hemostatic system with vitamin K in
the case of oral anticoagulants and protamine sulfate for
heparin. Patient outcome is often poor, with two-thirds usually
dying.
Thrombolytic drugs, particularly urokinase and tissue
plasminogen activator, are now being used more extensively,
particularly for treating coronary artery thrombosis. SICH has
been identified as a complication of these drugs, but
hemorrhagic transformation can occur after myocardial infarction
without the drugs, and the increased risk is fairly
small.
There is growing
interest in using thrombolytic drugs to treat cerebral vascular
occlusion. Treatment is
problematic since reversing the effect of the drug might
exacerbate the original thrombosis. Alcohol, if
used excessively, can predispose to SICH. This may be related to
its causing hypertension or altering coagulation mechanisms.
 Postoperative
Postoperative
Bleeding
after carotid endarterectomy, although it occurs in well under 1
percent of operations, may be devastating. Usually delayed a few
days, it occurs especially after opening a severely stenotic
artery (with hypoperfusion) particularly if the artery
supplied an area of previous infarction. Postoperative
hypertension with hyperperfusion exacerbates the risk, whereas
optimal control of blood pressure minimizes it. Postoperative
anticoagulants or antiplatelet agents increase the incidence.
Postcraniotomy bleeding probably relates to a number of
problems including inadequate hemostasis, low intracranial
pressure which minimizes tamponade, local and generalized DIC
unrecognized platelet abnormalities (including inhibition by salicylates), breakdown of autoregulation and postoperative
hypertension. In one series, such clots were seen in 0.5
percent of -1992 intracranial procedures: of these 24 patients,
8 died and 7 had a poor outcome. Special problems arise in
surgery for specific
lesions. Surgery for aneurysm may be
complicated by bleeding after imperfect clip placement, and
surgery for AVMs may be complicated by postoperative
circulatory breakthrough. SICH occurs after extracranial to
intracranial bypass surgery where there has been a prior
infarct. The risk of hematoma formation after
stereotactic surgery is 0 to 2.5 percent, but only one-quarter
of patients require surgery. Placement of monitoring devices
through the brain may lead to direct injury to vessels.
particularly in the face of DIC. Diagnostic procedures,
including lumbar puncture and angiography, and endovascular
techniques such as coiling or embolization for tumor or AVM, occlusion of
vessels or an aneurysm itself, and angioplasty for spasm may be
complicated by hemorrhage.
SICH after
cardiac operations may be related to a number of factors unique
to this kind of surgery. These include emboli, arterial
hypertension, increased venous pressure and anticoagulant use.
 Stroke
Stroke
After an
ischemic infarct, there may be transformation to a hemorrhagic
infarct or even frank parenchymal hemorrhage, presumably due to
reopening of the occluded vessel and leakage of blood from the
vessels damaged from the ischemic insult. Bleeding has been
seen in more than one-half of autopsied
patients.
Recent MRI studies have shown
some hemorrhage in 69 percent: several CT and MRI studies have
revealed small hematomas in about 15 percent and large hematomas
in 10 percent of patients although they were
often
asymptomatic. Risk is highest in patients with embolic strokes
from carotid or cardiac disease, those who have large infarcts
with significant mass effect and herniation, and those who have
early hypodense changes or areas of contrast enhancement on
CT. Anticoagulants predispose to this problem, and
their use in embolic disease of cardiac origin should be individualized.
This change is
usually not seen in the first day, but often occurs within 4
days, although a certain number occur later. Angiography has
revealed that many occlusions reopen within 2-1h, after
which reperfusion leads to this bleeding. Later bleeding may
relate to the development of collateral circulation. The development
of a parenchymatous hemorrhage
when
accompanied by clinical
deterioration has a poorer prognosis. Specific treatment
for this hemorrhage has not been discussed in detail. Since
heparin can exacerbate the bleeding (but not change the
incidence of hemorrhage), it
may
need to be stopped. Another
issue includes the trials of thrombolytic therapy for occlusive
stroke. It appears that, this treatment is efficacious
with
acceptable risk.
Venous and
sinus thrombosis, a complication of dehydration or congestive
heart failure, hematologic problems, oral contraceptives,
pregnancy, trauma, infection or malignancies including
leukemia may also cause SICH. Venous thrombosis may involve
the sagittal sinus, transverse sinus, cavernous sinus or
cortical veins. Clinical manifestations depend on the extent of
the thrombosis and
collaterals. There may be evidence of
elevated intracranial pressure with or without obvious focal
signs, depending on the sinus involved and the site of the
hemorrhage. Seizures may be a prominent event. Sagittal sinus
thrombosis can lead to SICH which
is usually in the parasagittal
white matter bilaterally. The CT and MRI appearance may be
diagnostic for such occlusions because of bilateral clots. There
may be a defect in filling of the sinus on contrasted CT or MRI
scans. Angiography can be helpful. Venography is not necessary
nor worth the risk. Treatment should be aimed at the underlying
condition. The use of anticoagulants in the face of a hematoma
is problematic. These hemorrhages have a significant mortality
rate.
 Post-Traumatic
Post-Traumatic
The
so-called delayed traumatic intracerebral hematoma (DTICH) is
discussed because it does occur spontaneously and differs from
other etiologies only in that the primary initiating factor, the
injury, occurs at a distinct point in time as opposed to being
the result of ongoing or progressive disease. There are
actually three groups of such hematomas, depending on the
vessel
of origin: (1) clots from
traumatic aneurysms on larger arteries, (2) classic DTICH from
smaller arteries, and (3) clots from venous injuries (see
above).
Traumatic
aneurysms can be caused by penetrating injuries or closed head
injuries (Table-9) and may be,
true, false
or mixed. At times
rupture, often fatal, occurs within days after injury. They
cause SICH in 10 percent of cases. The aneurysm may be detected
by comparing an uncontrasted CT scan with a contrasted CT scan.
Angiography should be performed if missiles or other objects
have passed near major arteries. Early prophylactic clipping is
suggested. Classic
DTICHs occur in 1.3 to 1.7 percent of patients with head injury
judged significant enough to perform CT and 2.3 to
8.4 percent of those
with Glasgow Coma Scale scores 8
and are
generally seen 3 to 4 days after injury. A variety of
mechanisms can play a role in their development (Table-10). As noted, decompressive surgery may contribute to
their formation by releasing tamponade in areas of
contusion. Treatment must be individualized. Prognosis
depends on the size and location of the clot and the
previous condition of the patient.
|
TABLE-9 Etiologies of Traumatic
Aneurysms |
|
I. |
Penetrating |
|
| |
Depressed fractures |
|
| |
Gunshot wounds |
|
| |
Knives. etc. |
|
| |
Iatrogenic |
|
|
II. |
Closed head injury |
|
| |
Tethering |
|
| |
|
Supraclinoid carotid |
| |
Local injury |
|
| |
|
Anterior cerebral at falx |
| |
|
Middle cerebral at sphenoid ridge |
| |
|
Posterior cerebral at tentorium |
| |
|
Cortical vessels at adhesions or in
linear fracture |
|
TABLE-10 Primary and Secondary
Factors Leading to Delayed Traumatic Intracerebral
Hematoma |
|
Vessel damage
|
|
Neuropil damage |
|
Vasospasm |
|
Vasodilation |
|
Vasoparalysis
|
|
Venous back
pressure |
|
Hypoxia,
hypotension |
|
Hypertension |
|
Medical
reduction of intracranial pressure |
|
Surgical
reduction of intracranial pressure |
|
Disseminated
intravascular coagulation |
|
Effects of
alcohol |
 Mycotic
Aneurysm
Mycotic
Aneurysm
SICH
after infection may be due to disruption of a
vessel wall, bleeding into
an infarction, or (most commonly) rupture of an aneurysm
arising from an infected vessel wall. Aneurysms
occur in perhaps 1.7 percent of patients with bacterial endocarditis, the most common cause,
and they are multiple in 20 percent. Neurological complications are seen in
up to one-third of patients with bacterial endocarditis,
one-half of which are vascular and more than one-half of
all victims die. Mycotic
aneurysms constitute 2.6 to 6 percent of
all aneurysms, but their incidence is thought to be
decreasing.
Although called "mycotic" aneurysms, most aneurysms of infectious origin are secondary to bacterial infections,
particularly subacute bacterial endocarditis. They are
caused by infected emboli that lodge in distal intracranial
arteries, particularly middle cerebral branches. Risk
factors include subacute endocarditis, intravenous drug
abuse, and immunosuppression. The type of infection is
changing as the pattern changes in endocarditis. Fungal
aneurysms have also been reported. They may also be the
result of infections external to a vessel such as septic
cavernous sinus
thrombophlebitis
and meningitis.
Mycotic
aneurysms present as SICH or subarachnoid hemorrhage, which
have a high mortality, or with just a headache.
Bleeding may occur within
1 to 2 days or up to months
after infection, but the average is about 17 days. CT
without and then with contrast enhancement or MRI may
reveal the aneurysm as well as the hemorrhage. The workup
should include complete cerebral angiography, which should be
repeated until treatment is finished. Initial treatment
should include antibiotics and correction of the cardiac
lesion if indicated. Decisions about intracranial surgery
should be based on the significance of the clot, details
about the aneurysm, and the response to antibiotics. Based
on review of the literature, it is
suggested:
1. If there is one distal middle cerebral artery aneurysm and
the patient has bled, excise the aneurysm. The artery of
origin will probably need to be sacrificed. An
extracranial to intracranial bypass may be required as an
adjunct.
2.
If
there is a proximal or unruptured aneurysm or an aneurysm on
a critical branch, treat with antibiotics and obtain serial angiograms, to see if the aneurysm is resolving, stable,
or enlarging. Consider excising enlarging aneurysms and
follow
healing aneurysms until they disappear. The appropriate
frequency for angiograms is not well established, but
probably every 10 to 14 days is reasonable. A significant
proportion of aneurysm will not disappear, but their walls will be
stronger after they have had time to develop fibrosis.
3. Individualize if there are multiple aneurysms.
Endovascular occlusion has also been used successfully.
Hemorrhage may occur with encephalitis, specifically Herpes
simplex
encephalitis
and with brain abscess. SICH may also occur in a variety of
circumstances in patients who are immunosuppressed, particularly by
AIDS.
 Childhood
Childhood
The
most unique hemorrhage in childhood is the intraventricular
and periventricular hemorrhage primarily seen in the
prernature. Other predisposing conditions, such as vein of Galen
malformations, leukemia and idiopathic thrombocytopenic purpura and inherited coagulation disorders may be seen
especially in childhood.
 Pregnancy
Pregnancy
Intracranial hemorrhage, including SICH, is the leading nonobstetrical cause of maternal mortality in pregnancy. It
may be related to normal changes in cardiovascular
physiology, complications of pregnancy including
hypertension in toxemia and eclampsia, coagulation
disorders and bleeding from preexisting lesions. Routine
nonoperative and operative care are indicated, although it
should be remembered that mannitol can dehydrate the fetus,
hypotension can be detrimental, and anticonvulsants have teratogenic and depressant effects. Method of delivery does
not influence bleeding and should be decided on obstetrical
grounds